Over the past two decades the use of antidepressants has skyrocketed. Among the highest users in the world are Canadians, with over 3 million Canadians on a depression-fighting drug such as prozac or zoloft. This is perhaps not so surprising in today’s high-stress society, where long days are spent inside a school or office, and even the smallest sign of depressed mood justifies a visit to the doctor. Whether the steep rise in depression is due to greater incidence or simply a result of increased awareness, more people are being diagnosed with depression than ever before. With over 80% of physician visits for depression resulting in a prescription for antidepressants (CWHN, 2010), many people are relying on these drugs to relieve their depressive symptoms and function on a day-to-day basis.
While all antidepressant drugs have some negative side effects, they usually provide sufficient therapeutic benefits that counteract the associated harms. But what happens when depression is resistant to the benefits of antidepressants? This is the case for patients with treatment-resistant depression (TRD). For these patients, which represent more than one third of depressed patients (Christopher et al, 2012), treatment with antidepressants is rarely successful. They also show little improvement with intense psychotherapy, such as cognitive behavioural therapy. TRD is associated with significantly greater disability and suffering, and is more likely to result in suicide than other forms of depression. In a society where treating depression revolves around antidepressants, individuals with TRD may need to seek more extreme-and dangerous-forms of treatment such as deep-brain stimulation (DBS).
Over the past decade, advances in brain-computer interface technology have lead to the development of DBS. Due to the current lack of treatment for TRD, a large amount of attention has turned to DBS for potential therapy.
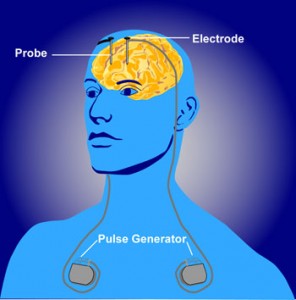
A DBS device consists of four components: (a) a stimulating lead, which is surgically implanted within the desired brain target and is equipped with electrodes that deliver therapeutic stimulation, (b) a locking device to anchor the lead, (c) a programmable pulse generator that delivers the therapeutic current, and (d) an extension cable that connects the pulse generator to the lead. The device works by stimulating certain regions in the brain that are thought to be dysfunctional in depression. It is programmed to enhance the therapeutic effect for the user, adjusting stimulation parameters to provide an optimal level of symptom relief. The patient is then given a hand-held device to take home so that they can control the level and time of stimulation, albeit to a limited and safe extent.
Due to it’s reversibility and adjustability, DBS has become the standard of care for some other diseases of the brain, including medication-refractory Parkinson’s disease (PD), essential tremor and dystonia (a disorder characterized by involuntary muscle contractions). Indeed, DBS has proven to be successful in some PD patients, attenuating motor symptoms such as tremors and difficulty initiating movement, thereby allowing them to move freely.
Is it possible for DBS to have the same level of success for TRD? Unfortunately, the clinical impact of DBS for TRD remains poorly understood. At present, DBS for the majority of psychiatric disorders is not yet FDA (Food and Drug Administration) approved and many clinical trials for DBS in psychiatric patients are still in the early-phase. But there seems to be good reasons for this. Not only could DBS result in profoundly negative consequences for the user, but in the case of TRD, one can argue that DBS is completely unethical. This is because the lack of established evidence that DBS is effective for treating TRD makes it difficult to predict which patients will benefit from DBS and which will not.
The most daunting risk of DBS is that of stroke or hemorrhage during implantation of the device. Although it is unlikely to occur, too few procedures have been performed to reliably determine the level of risk for patients with TRD. Another major concern of DBS surgery is device related infection, which occurs in 0%-15% of cases (Clausen, 2010). Luckily, these infections are often readily treated by device removal and antibiotic therapy. Infection rates will hopefully be reduced with the future development of smaller implants.
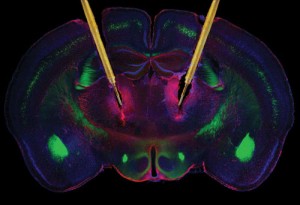
Stimulating electrodes in the brain
Overall, long-term implantation of DBS devices is well tolerated and positive responses have been reported in over 50% of patients with TRD (Goodman and Alterman, 2012). Unfortunately, not everyone with TRD will be able to experience these benefits due to financial reasons. The current cost of DBS surgery and hardware is about $80 000 (Goodman and Alterman, 2012). This is a steep price to pay for an operation and device with so many unknown complications. DBS also requires lifelong upkeep and involves periodic surgery to replace the batteries of the pulse generator. For the patients who do not respond to DBS, the procedure may be more of a burden than a help.
What makes the use of DBS in patients with TRD especially precarious is the risk of therapeutic misconception. TRD patients may be so desperate for treatment that they would be willing to do anything to receive help, even if they are aware of the many risks associated with DBS. This is especially problematic in early-stage research trials for DBS, where subjects with TRD might mistake their participation in the trial as therapeutic in nature.
Optimal DBS targets for TRD remain uncertain
A second problem in the case of TRD is the uncertainty of effective brain targets for DBS. In contrast to the more than 75 000 DBS implants that have been performed worldwide for movement disorders, DBS has now been performed in fewer than 100 TRD patients. It is therefore not surprising that the optimal therapeutic target sites in the brain are less well defined for TRD than they are for movement disorders, leaving more room for error. This situation is not helped by the fact that the pathophysiology (functional changes that accompany a disorder) of major psychiatric disorders remains elusive. Although rodent models for depression exist, extrapolating these models to human neuro-circuitry is a huge challenge.
In the end, one has to carefully weigh the risks and benefits of DBS to decide whether or not to undergo the procedure or participate in clinical trials. A person with TRD who has few other treatment options would most likely be in favour of DBS. But like any novel technology, especially those with implications for human health, DBS needs to be approached with skepticism. Amidst the exciting prospect of treating psychiatric disorders such as TRD, it is important that the negative impacts of DBS are thoroughly discussed with potential users. While doctors and researchers are inclined to do what is best for their patients and subjects, they may not always be fully equipped to protect them from harm in the face of new and unpredictable medical interventions. That being said, the medical community is not ready to give up on restoring patients with refractory psychiatric disorders back to health. As long as DBS research continues, there is the prospect of new and better treatments for patients with otherwise untreatable conditions.